Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(2); 2024 > Article
-
Review ArticleDiabetes, obesity and metabolism Young-Onset Diabetes in East Asians: From Epidemiology to Precision Medicine
 Keypoint
Keypoint
· Young-onset diabetes, diagnosed before the age of 40, affects 1 in 5 adults diagnosed with diabetes in Asia.
· Family history, beta-cell dysfunction, obesity, and a high burden of kidney disease and mental illness are key characteristics of patients with young-onset diabetes.
· Care reorganization is needed to deliver data-driven, multidisciplinary, and personalized care guided by biogenetic markers aimed at improving the precision of diagnosis and classification in order to reduce the growing burden of young-onset diabetes. -
Juliana C.N. Chan
 , Chun-Kwan O, Andrea O.Y. Luk
, Chun-Kwan O, Andrea O.Y. Luk -
Endocrinology and Metabolism 2024;39(2):239-254.
DOI: https://doi.org/10.3803/EnM.2024.1968
Published online: April 16, 2024
Department of Medicine and Therapeutics, Hong Kong Institute of Diabetes and Obesity and Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong
- Corresponding author: Juliana C.N. Chan. Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong Tel: +852-35053138, Fax: +852-35053138, E-mail: jchan@cuhk.edu.hk
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,087 Views
- 29 Download
- ABSTRACT
- INTRODUCTION
- YOUNG-ONSET DIABETES – A SILENT KILLER
- YOUNG-ONSET DIABETES: INSIGHTS FROM PIMA INDIANS
- IMPORTANCE OF USING THE ORAL GLUCOSE TOLERANCE TEST TO DETECT HYPERGLYCAEMIA
- INSULIN RESISTANCE AND BETA-CELL DYSFUNCTION IN YOUNG-ONSET DIABETES
- FAMILIAL HERITABILITY AND GENETICS OF YOUNG-ONSET DIABETES
- GESTATIONAL DIABETES, IN UTERO EXPOSURE TO HYPERGLYCAEMIA AND CHILDHOOD OBESITY
- MULTICAUSALITY OF YOUNG-ONSET DIABETES
- YOUNG-ONSET DIABETES AND CHRONIC KIDNEY DISEASE
- THE COMORBIDITIES OF YOUNG-ONSET DIABETES CALL FOR EARLY DIAGNOSIS, PREVENTION, AND TREATMENT
- CLOSING THE IMPLEMENTATION GAP TO IMPROVE OUTCOMES IN YOUNG-ONSET DIABETES
- USING A MULTI-COMPONENT STRATEGY TO REDEFINE YOUNG-ONSET DIABETES FOR PRECISION CARE
- CONCLUSIONS
- Article information
- References
ABSTRACT
- Precision diagnosis is the keystone of clinical medicine. In East Asians, classical type 1 diabetes is uncommon in patients with youngonset diabetes diagnosed before age of 40, in whom a family history, obesity, and beta-cell and kidney dysfunction are key features. Young-onset diabetes affects one in five Asian adults with diabetes in clinic settings; however, it is often misclassified, resulting in delayed or non-targeted treatment. Complex aetiologies, long disease duration, aggressive clinical course, and a lack of evidence-based guidelines have contributed to variable care standards and premature death in these young patients. The high burden of comorbidities, notably mental illness, highlights the numerous knowledge gaps related to this silent killer. The majority of adult patients with youngonset diabetes are managed as part of a heterogeneous population of patients with various ages of diagnosis. A multidisciplinary care team led by physicians with special interest in young-onset diabetes will help improve the precision of diagnosis and address their physical, mental, and behavioral health. To this end, payors, planners, and providers need to align and re-design the practice environment to gather data systematically during routine practice to elucidate the multicausality of young-onset diabetes, treat to multiple targets, and improve outcomes in these vulnerable individuals.
- According to the noncommunicable disease (NCD) Risk Factor Collaboration, between 1980 and 2014, the age-standardized prevalence of diabetes in adults aged 20 to 64 years increased from 3.2% to 7.8% in men, and from 3.9% to 6.8% in women [1]. In a nationwide study in Korea, from 2006 to 2015, the overall incidence of diagnosed diabetes decreased by 0.1% per year, but increased from 0.5 to 0.7 per 1,000 individuals in the 20 to 29 age group and from 2.0 to 2.6 per 1,000 individuals in the 30 to 39 age group. Importantly, the proportion of obese young adults with diabetes increased from 51.4% in 2006 to 72.4% in 2015 [2].
INTRODUCTION
- In a simulation model based on a territory-wide diabetes surveillance database curated from electronic medical records (EMRs) including 2,608,973 individuals followed from 2001 to 2019, researchers from Hong Kong estimated that a 20-year-old Chinese individual with prediabetes would live with diabetes for 32.5 years—or 51.6% of his or her remaining life years—with diabetes, versus the corresponding figures of 12.7 years and 18.4% in an individual with normoglycaemia at 20 years old [3].
- In a Korean national database including 2,101,599 young adults aged 20 to 39 years without prediabetes, diabetes, or cardiovascular disease (CVD) at baseline who were followed up for 10 years, incident diabetes, defined by fasting plasma glucose (PG), was associated with a hazard ratio (HR) of 1.60 for all-cause death and 1.13 for CVD [4]. In a pooled analysis of 22 prospective studies of the Asia Cohort Consortium, which included 1,002,551 Asian individuals aged 30 years or above, conducted between 1963 and 2006, 148,868 deaths were ascertained after a median follow-up period of 12.6 years. Individuals with diabetes had a 1.89-fold higher risk of all-cause death than those without diabetes; notably, the HR decreased from 2.43 in the <50 age group to 1.51 in the ≥70 age group [5].
- Using data from the Hong Kong Diabetes Register [6], researchers from Asia first reported a high risk of premature death in patients with young-onset diabetes. In 9,509 Chinese patients with type 2 diabetes who were followed up for 7.5 years, 21% of whom were diagnosed before the age of 40 years, patients with young-onset diabetes had age- and sex-adjusted HRs of 1.48 for CVD and 1.35 for chronic kidney disease (CKD) compared to patients with later-onset diabetes, despite an age difference of 20 years. These hazards were attenuated after adjustment by disease duration, highlighting its importance in driving the development of complications [7].
- On average, type 2 diabetes was found to shorten the lifespan by at least 4 years [8], and the loss of life expectancy was increased to 14 years or more if individuals were diagnosed at a young age [9,10]. Other meta-analyses have also reported a high risk of premature death associated with youth-onset diabetes (age of diagnosis <20 years) [11] and young age of diagnosis [12]. In the latter report, which included 26 observational studies comprising 1,325,493 individuals from 30 countries, the risk of all-cause death and macrovascular and microvascular disease was negatively associated with age at diagnosis of diabetes. For every 1-year increase in age at diagnosis, there were 4%, 3%, and 5% decreased risks of all-cause death, macrovascular disease, and microvascular disease, respectively, adjusted for current age [12].
YOUNG-ONSET DIABETES – A SILENT KILLER
- In the early 1970s, the high prevalence of type 2 diabetes and obesity in Pima Indians provided a glimpse of the impact of rapid acculturation on the development of metabolic diseases in non-European populations. In a recent review of 40 years of research among Pima Indians [13], the authors summarized the epidemiological lessons learnt from this population, which shared many similarities with diabetes in Asians. These include the rarity of autoimmunity in young people with diabetes, in whom genetics, gestational diabetes mellitus (GDM), obesity, beta-cell dysfunction, and kidney disease are key features [14-17].
YOUNG-ONSET DIABETES: INSIGHTS FROM PIMA INDIANS
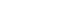
- One of the key observations in Pima Indians was the bimodal distribution of 2-hour PG levels during a 75-g oral glucose tolerance test (OGTT) in adults aged 25 to 65 years. These findings suggested that during a glucose challenge, a group of individuals had a high blood glucose response, which formed the basis of impaired glucose tolerance (IGT) (Fig. 1) [18]. A prospective analysis in Pima Indians confirmed the associations of high 2-hour PG during the OGTT with progression to type 2 diabetes and its complications, including retinopathy and albuminuria [13].
- Precise diagnosis is the keystone of clinical medicine. It has long been recognized that up to 50% of Asians with diabetes are diagnosed by 2-hour PG during OGTT [19]. In non-Europeans, there are pitfalls in using glycated haemoglobin (HbA1c) alone to diagnose diabetes or prediabetes. This is particularly true for Asians, who not uncommonly have hemoglobinopathy, which can confound HbA1c values [20,21]. In a recent analysis of more than 80 contemporary studies among adults aged 20 to 79 years, the global prevalence of IGT exceeded that of impaired fasting glucose (IFG) in 2021 (9.1% vs. 5.8%), suggesting that a substantial number of individuals could be missed if only fasting PG or HbA1c were used to diagnose prediabetes for prevention purposes [22].
- In the late 1990s, two community-based prospective cohorts (the Hong Kong Family Diabetes Study [HKFDS] and Better Health for Better Hong Kong [BHBHK] Survey) were established in a workforce in Hong Kong to study gene-environment interactions in diabetes. In these young participants, with a mean age of 40 years, over 50% of individuals with prediabetes or diabetes were diagnosed by 2-hour PG during OGTT [23-25]. There is now confirmatory evidence showing that the progression from IGT to diabetes can be prevented by structured lifestyle intervention and drugs such as metformin and acarbose [9]. However, these benefits were not observed in individuals with isolated IFG [26]. Thus, OGTT shall remain the gold standard for the detection, prevention, and treatment of prediabetes and diabetes, especially in young individuals with risk factors [27,28].
IMPORTANCE OF USING THE ORAL GLUCOSE TOLERANCE TEST TO DETECT HYPERGLYCAEMIA
- The high prevalence of type 2 diabetes in Pima Indians was in part driven by the high prevalence of obesity, which was associated with hepatic insulin resistance—a condition accompanied by increased hepatic glucose production and reduced clearance of insulin. However, irrespective of the presence of obesity, individuals who developed diabetes or IGT exhibited a loss of acute-phase insulin secretion during OGTT, suggesting the importance of beta-cell dysfunction in these individuals [29]. In this light, the importance of beta-cell dysfunction in Asians with type 2 diabetes has long been highlighted as a feature in contrast to Europeans [30-32]. In a 10-year community-based study in Korea, although insulin resistance increased with age, often accompanied by increased insulin secretion, individuals with an insufficient insulin response went on to develop diabetes [33].
- A cross-sectional analysis of 5,170 Chinese patients with type 2 diabetes found negative associations of beta-cell function indicated by homeostasis model assessment (HOMA2-beta%) with a family history of diabetes, the number of affected family members, and body mass index (BMI). The slope of the decline in HOMA2-beta% by diabetes duration was also greater in individuals with young-onset diabetes than in those with later-onset diabetes amongst individuals with a BMI <27.5 kg/m2 (Fig. 2) [34].
- Using prospective data from the Hong Kong Diabetes Register, which was established in 1995 as a research-driven quality improvement program with detailed documentation of baseline clinical profiles, laboratory tests, medications, hospitalization diagnosis, and causes of death [6,35], researchers reported a consistently higher HbA1c level and its steep upward trajectory, likely related to rapid beta-cell deterioration, in the first decade after diagnosis in individuals with young-onset diabetes, which translated to a very large glycaemic burden over time [36].
INSULIN RESISTANCE AND BETA-CELL DYSFUNCTION IN YOUNG-ONSET DIABETES
- In monozygotic twins, the concordance for the lifetime risk of type 2 diabetes approached 100%, compared to 50% for type 1 diabetes [37]. The attenuated risk of type 2 diabetes amongst Pima Indians with genetic admixture [13], as well as the strong heritability of insulin secretion and body fat in family-based studies [38,39], lent support to the importance of genetics in type 2 diabetes. In the HKFDS, which included 179 families with 913 individuals, 78% of families had at least one individual with diabetes diagnosed at the age of 40 years or younger. Amongst these families with young-onset diabetes, 25% of siblings had metabolic syndrome, and the study found high heritability for diabetes, hypertension, central obesity, insulin resistance, and beta-cell function of 0.4 to 0.6 [24]. In the Botnia Family Study, the heritability of type 2 diabetes was 0.69 in individuals diagnosed at 35 to 60 years, which dropped to 0.31 in those diagnosed at 35 to 75 years [40]. In Hong Kong Chinese, a family history of young-onset diabetes was associated with a 6- to 8-fold increased risk of incident diabetes versus less than a 1.6-fold increased risk for a family history of diabetes diagnosed after the age of 50 years compared to no family history of diabetes [25].
- More than two decades of global efforts using genome-wide association studies (GWAS), whole-exome sequencing (WES), and whole-genome sequencing have implicated common and rare variants in coding and non-coding regions of hundreds of loci across the human genome as playing causal roles in the onset and progression of type 2 diabetes [41]. However, the majority of participants in these studies had European descent, raising the issue of the generalisability of these results to non-European populations. Indeed, similar studies in Asian populations have revealed inter-ethnic differences in genomic architecture, as well as in the location, frequency, and effect sizes of these risk alleles [42]. In the latest meta-analysis of GWASs, including data from 77,418 individuals with type 2 diabetes and 356,122 healthy control individuals from Asia, the authors identified 61 new loci associated with diabetes implicated in pancreatic, adipose, and muscle biology not found in European populations [43].
- Considering potential aetiological differences between young-onset diabetes and later-onset diabetes, only a few studies have specifically addressed genetic associations stratified by age of diagnosis [44-46]. In a recent GWAS of the UK Biobank stratified by the age of diagnosis (<50, 50–60, 60–70, and >70 years), researchers discovered three independent single nucleotide polymorphisms (SNPs) mapped to transcription factor 7 like 2 (TCF7L2) in patients diagnosed less than 50 years, and another 17 SNPs identified in the overall GWAS displayed differences in effect size dependent on age of diagnosis. In another analysis of the UK Biobank stratified by BMI and age of diagnosis, researchers reported 18 SNPs that showed subgroup differences, including one in neurogenin-3 (NEUROG3), a gene linked to maturity-onset diabetes of the young (MODY) [45].
- In this light, nearly 40 subtypes of monogenic diabetes, including MODY, have been reported [47]. In the largest WES study, including 20,791 individuals with type 2 diabetes and 24,440 control participants from five ancestries, rare variants located in pancreatic and duodenal homeobox 1 (PDX1), glucokinase (GCK), and HNF1 homeobox A (HNF1A) were associated with 1.5 to 3.5 increased odds of type 2 diabetes [48]. In family-based studies, common variants of monogenic diabetes have been shown to modulate the age of diagnosis of MODY. For example, the common HNF1A variant I27L was associated with a younger age of diagnosis of HNF1A-MODY with protein-truncating mutations [49]. On the other hand, polygenic risk scores of the common form of type 2 diabetes also jointly advanced the age of diagnosis of HNF1A-MODY [50]. We and others have reported that common variants of type 2 diabetes, including some located in genes for monogenic diabetes and MODY, independently predicted a younger age of diagnosis of type 2 diabetes and earlier insulin requirement in both Caucasians and Asians [51,52]. By using patients with familial young-onset diabetes as cases in the discovery cohort, researchers from Asia have reported genetic associations of dachshund family transcription factor 1 (DACH1) [53], paired box 4 (PAX4) [54], carboxypeptidase E (CPE), and insulin degrading enzyme (IDE) [55] with type 2 diabetes. These genes are strongly implicated in islet biology, in keeping with the importance of beta-cell dysfunction in Asian patients with young-onset diabetes [30].
FAMILIAL HERITABILITY AND GENETICS OF YOUNG-ONSET DIABETES
- In Pima Indians, in utero exposure to hyperglycaemia was associated with a high risk of youth-onset obesity and type 2 diabetes in offspring born to women with GDM. This was in contrast to the low risk of diabetes in offspring born to the same woman during her other uncomplicated pregnancies [56,57]. In a prospective cohort of Chinese women and their offspring recruited to the Hyperglyacemia and Adverse Pregnancy Outcome (HAPO) Study, offspring born to women with GDM had a higher incidence of abnormal glucose tolerance and overweight/obesity, as well as higher BMI, blood pressure and lower oral disposition index and a trend toward reduced beta-cell function than offspring born to women without GDM. For each standard deviation increase in maternal fasting, 1-hour, and 2-hour PG during OGTT at 24 and 32 weeks of the index pregnancy, there was a corresponding increase in the adjusted odds ratio of 1.85 to 2.00 for abnormal glucose tolerance in the offspring [58].
- In a systematic review on GDM, Asian women had the highest risk of developing GDM amongst all populations. These women were at high risk of developing young-onset diabetes, and their children also had a high risk of adiposity in later life [59]. In a meta-analysis including 71,998 children and 353,513 adolescents from Asia, the pooled prevalence for obesity was 5.8% in children aged 5 to 11 years and 8.6% in adolescents aged 12 to 19 years [60]. In a school-based survey including 2,115 Chinese adolescents from Hong Kong, the prevalence of metabolic syndrome using the criteria for Asian adults was 2.4%, with 32.2% having hypertension; 10.9%, increased triglycerides; 9.0%, central adiposity; 2.4%, low high-density lipoprotein cholesterol; 0.3%, IFG and 17%, increased albuminuria. Overweight (adjusted odds ratio, 32.2), a positive family history of diabetes (4.3) and studying at schools of lower academic grading (5.5) were independent risk factors, suggesting the importance of nature and nurture in youth-onset metabolic syndrome [61].
GESTATIONAL DIABETES, IN UTERO EXPOSURE TO HYPERGLYCAEMIA AND CHILDHOOD OBESITY
- Age, obesity, and family history are major risk factors for diabetes [62]. Thus, individuals who develop diabetes at young age, especially if lean, require comprehensive profiling to elucidate additional aetiologies. In a recent review article [63], we summarized the multiple challenges in the diagnosis and management of young-onset diabetes, which affects one in five adult patients with type 2 diabetes in clinic settings in Asia [64]. Based on 30 years of data collection in real-world practice accompanied by a biobank with linkage to hospitalization data, we have reported that 8% of patients diagnosed with type 2 diabetes before the age of 40 years had positive anti-islet autoantibodies measured in stored serum, suggesting a diagnosis of latent autoimmune diabetes in adults (LADA). Compared to patients with classical type 1 diabetes, these young patients with LADA had a 2.8-fold increased risk of end-stage kidney disease (ESKD), in part due to missed diagnosis and delayed insulin treatment [65]. On the other hand, 3% to 5% of these young patients might have monogenic diabetes or MODY, and if diagnosed early, those individuals might benefit from sulphonylureas or dipeptidyl peptidase 4 inhibitors in the presence of residual beta-cell function [16,66]. However, these subtypes of diabetes cannot fully explain the low beta-cell function [34] and poor glycaemic control despite high usage of insulin [67-69], as well as the high prevalence of family history of diabetes in the majority of young patients [15,17].
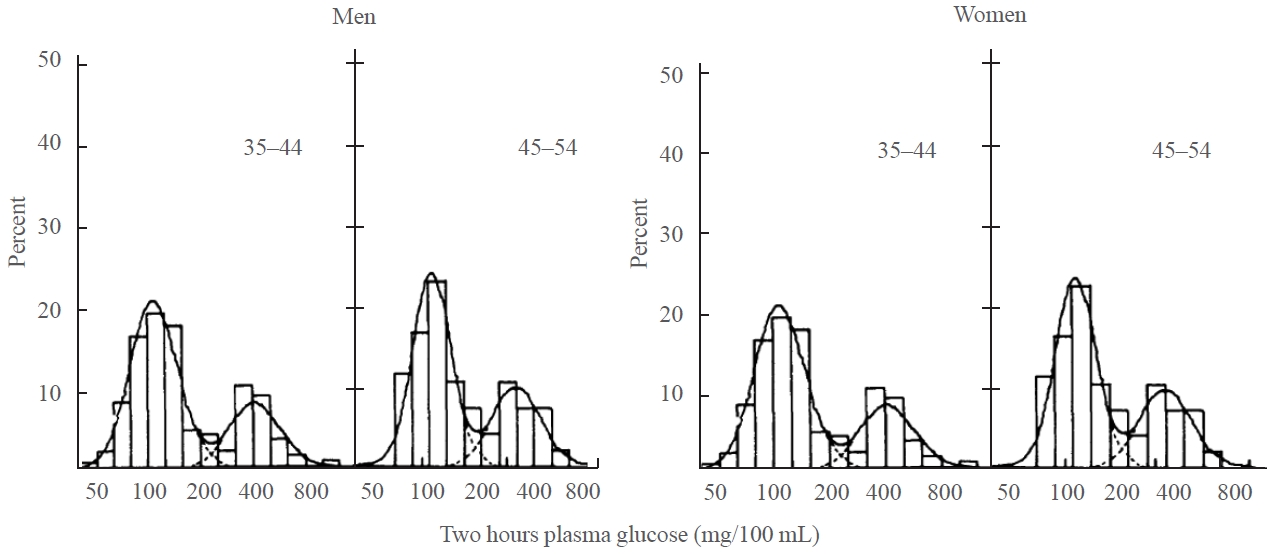
- Taking a life-course approach and considering other family, personal, and environmental factors, Fig. 3 summarizes the myriad of factors that may contribute to the development of young-onset diabetes, calling for further investigations to define the causes, trajectories, and consequences of young-onset diabetes [63]. To this end, diabetes is a typical example of multicausality, an important concept where a disease may occur due to different causal mechanisms made up of multiple components, with each component having different strengths, and the clinical manifestation will depend on interactions across these components (Fig. 3) [70,71].
- Given the differences in genetic architecture, ethnicity, ecosystem, cultures, lifestyles, and healthcare systems across different populations, all of which can contribute to the predisposition, precipitation, and perpetuation of diabetes [72], there is a need to conduct more population-specific investigations based on families and prospective cohorts with multidimensional data, including not just multiomic data, but also data on demographic and biomedical-psychosocial-behavioral factors, and—most importantly—access to and quality of care to allow the use of big data to unravel missing aspects of the heritability of young-onset diabetes and, evaluate the effects of interventions on outcomes in order to bring precision medicine into clinical practice [63,73].
MULTICAUSALITY OF YOUNG-ONSET DIABETES
- In the 1970s, Pima Indians with diabetes diagnosed before the age of 25 years had a high incidence of ESKD after 15 to 20 years, which was the leading cause of death [74]. Similar patterns were reported in Aboriginal Australians with type 2 diabetes [75]. In Japanese [76,77] and Chinese populations, patients with young-onset diabetes had a higher incidence of nephropathy than their counterparts with type 1 diabetes [78]. In the follow-up study of the World Health Organization Multinational Study of Vascular Disease in Diabetes (WHO MSVDD) including 10 countries, the authors reported marked inter-country variations in the incidence of complications. While Chinese, Japanese, and Native American patients with type 2 diabetes had a very low incidence of coronary arterial disease but a high incidence of ESKD, the reverse was observed in European patients [79].
- In Hong Kong, the rising incidence of both type 1 diabetes and type 2 diabetes in Chinese under the age of 40 years [80] concurred with the most rapid rate of increase in kidney replacement therapy in the 45 to 65 age group [81]. In Korea, amongst 84,384 patients with type 2 diabetes with 5.16 years of follow-up, patients with young-onset diabetes had 1.70 odds ratios of developing CKD compared to those with later-onset diabetes after adjusting for clinical variables [82]. Although disease duration is one of the key drivers of ESKD, in the Hong Kong Diabetes Register, the increased risk of CKD with longer diabetes duration decreased with an older age at diabetes diagnosis. For every 5-year increase in diabetes duration, the adjusted HR for CKD was 1.37 in patients diagnosed at 20 to 29 years versus 1.01 in those diagnosed at ≥70 years, highlighting the vulnerability of these young patients to ESKD [83].
- The close link between CKD and CVD has been highlighted in many practice guidelines, which recommend the use of estimated glomerular filtration rate (eGFR) and urinary albumin creatinine ratio (ACR) as time-tested biomarkers for vascular and kidney health [84]. In a set of risk equations developed from the prospective Hong Kong Diabetes Register, different combinations of clinical and cardiometabolic parameters predicted coronary arterial disease, stroke, ESKD, heart failure, and all-cause death in Chinese patients with type 2 diabetes. However, eGFR and ACR, which are closely associated with a long disease duration due to a young-onset of diabetes, were common parameters in all risk equations [6]. To this end, there is now a global consensus on the importance of cardiovascular-kidney-metabolic syndrome, for which a global risk assessment—including eGFR and ACR, along with other biomarkers, notably HbA1c and low-density lipoprotein cholesterol—should be measured at least annually. This is particularly important for patients with young-onset diabetes, who are at high risk of heart and kidney disease [85].
YOUNG-ONSET DIABETES AND CHRONIC KIDNEY DISEASE
- The epidemiological data gathered over four decades support the importance of familial factors including genetic and possibly epigenetic influences, secondary to early and prolonged exposure to hyperglycaemia and metabolic insults, which contribute to poor outcomes in patients with young-onset diabetes [9]. In the clinic-based Joint Asia Diabetes Evaluation (JADE) Register including 41,029 patients from nine countries in Asia, one in five adults with diabetes in Asia had young-onset diabetes diagnosed before the age of 40 years. Compared to their older-onset counterparts, these patients had worse cardiometabolic risk factors and were less likely to receive organ-protective drugs despite the presence of complications [64].
- Given the universal health coverage with provision of medical care by the Hong Kong Hospital Authority, which operates all publicly-funded clinics and hospitals, researchers from Hong Kong have tracked diabetes-related trends and outcomes in the territory since 1995. The Hong Kong Diabetes Surveillance Database included EMRs from 4 million people receiving publicly-funded medical care, of whom 0.8 million had diabetes based on disease codes, laboratory results, or their use of medications. More than 50% of them had undergone structured assessments, based on the protocol of the Hong Kong Diabetes Register developed by academics as a research-driven quality improvement program since 1995 [35,86]. Using data from this database and register, we reported a 2- to 6-fold increased risk for hospitalization in patients with young-onset diabetes, notably for kidney disease, mental illness, and infections [87,88]. Despite a 50% to 80% decline in all-cause and cause-specific death rates between 2001 and 2016 in the overall population, the standard mortality ratio in the 20 to 44 age group fluctuated between 4.92 and 7.86 [89]. During the same period, while the proportion of patients with poor glycaemic control (HbA1c ≥9%) fell in all age groups, patients with young-onset diabetes continued to have the worst glycaemic control despite having access to many glucose-lowering drugs (Fig. 4) [69].
- Apart from an incomplete understanding of the aetiologies of young-onset diabetes, the missed diagnosis of diabetes subtypes (e.g., LADA and MODY) requiring specific treatment, the random nature of management in the absence of evidence [90], the aggressive clinical course with rapid beta-cell failure compounded by poor adherence, suboptimal self-management, and psychosocial stress can all contribute to the poor outcomes in these young individuals [63].
- The ongoing International Diabetes Management Practices Study (IDMPS) aimed to detect trends and quality of care practices outside North America and Western Europe. In an analysis of data from 21 countries, 30% to 40% of patients reported depressive symptoms using the Patient Health Questionnaire (PHQ-9), with 8% to 16% having moderate depression indicated by a PHQ-9 score ≥10. Female gender, complications, poor glycaemic control, and low socioeconomic status were independently associated with depressive symptoms [91].
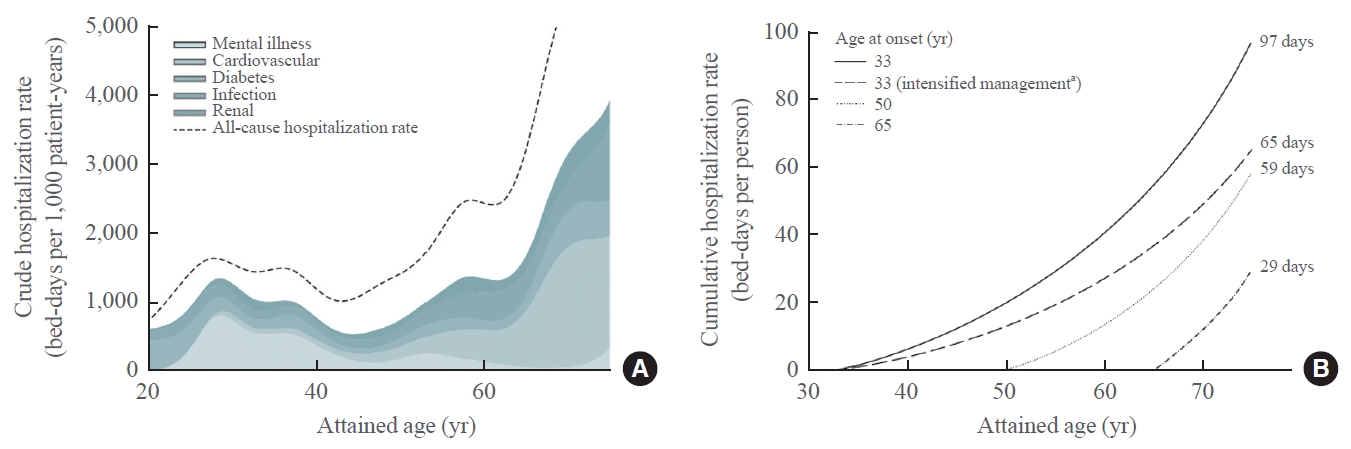
- Using EMR data in patients with diabetes, researchers from Singapore reported that the highest users of tertiary healthcare services were clusters of young women with short-to-moderate disease duration and comorbid depression, as well as older patients with moderate-to-long disease duration and multiple morbidities [92]. Using register and EMR data from 0.42 million Chinese adults with incident type 2 diabetes observed between 2002 and 2014, we estimated that patients with young-onset diabetes spent an average of 100 hospital days from diagnosis to age of 75, with one-third of the hospitalizations due to mental illness before the age of 40. We further estimated that by delaying the onset of diabetes or optimizing control of all cardiometabolic risk factors, the hospitalization rates in patients with young-onset diabetes could be reduced by 30% to 60% (Fig. 5) [87].
THE COMORBIDITIES OF YOUNG-ONSET DIABETES CALL FOR EARLY DIAGNOSIS, PREVENTION, AND TREATMENT
- After nearly half a century of research, high-level evidence now shows that type 1 and type 2 diabetes, as well as their complications, are preventable and treatable. For autoimmune type 1 diabetes, teplizumab, a humanized monoclonal antibody to CD3 on T cells, has been approved in the United States to delay the onset of clinical type 1 diabetes in relatives of patients with type 1 diabetes and improve beta-cell function in patients with newly diagnosed type 1 diabetes [93,94]. The availability of these preventive treatments strongly supports the inclusion of testing for auto-islet antibodies and beta-cell function in the risk assessment and management of young-onset diabetes.
- For type 2 diabetes, multiple studies from different populations including Asians have confirmed that structured lifestyle interventions and medications such as metformin and acarbose could delay the onset of diabetes [9]. These early prevention programs for type 2 diabetes are particularly effective [95] and cost-effective [96] in young people with IGT. Importantly, the results of these early prevention efforts are expected to translate to a long-term reduction in microvascular and macrovascular complications and all-cause death [97].
- A randomized controlled trial found that a weight reduction of 15 kg could cause remission of type 2 diabetes for up to 2 years in obese patients with type 2 diabetes [98]. However, in a recent analysis of the Hong Kong Diabetes Surveillance Database including 37,326 individuals with newly diagnosed type 2 diabetes followed in 2002 to 2017, only 6.1% achieved diabetes remission, with an overall incidence rate of 7.8 per 1,000 person-years. Despite this low rate of remission in real-world practice, a greater weight reduction in the first year of diagnosis was associated with an increased likelihood of remission, and any period of disease remission was associated with a reduced death rate [99].
- On the other hand, early glycaemic control in patients with type 2 diabetes, often using combination drugs, improved glycaemic durability and reduced the likelihood of treatment escalation including insulin, especially in patients with a young age of diagnosis [100,101]. In the Japan Diabetes Optimal Integrated Treatment study for 3 major risk factors of cardiovascular diseases (J-DOIT3) study, using commonly used glucose-lowering drugs to treat patients intensively to multiple targets, supplemented by self-management and education, was associated with extremely low events, with no cases of ESKD after 8 years [102]. Importantly, both interventional [103] and observational studies [104] have shown that early glycaemic control had legacy effects that translated to long-term reductions in complications and death.
- Fig. 6 provides a conceptual framework explaining how individuals with different beta-cell capacities, likely to be endowed at birth, might present with diabetes at different ages given the same trajectory of beta-cell failure under the same metabolic stress. Given the same beta-cell capacity, different combinations of stress factors may cause different trajectories of beta-cell function, with different ages of diagnosis reflecting the significant loss of beta-cell function. By changing lifestyle and using medications to control multiple risk factors, it is possible to preserve beta-cell function and delay the onset of diabetes, treatment escalation (including insulin use), and eventually the development of complications (Fig. 6).
CLOSING THE IMPLEMENTATION GAP TO IMPROVE OUTCOMES IN YOUNG-ONSET DIABETES
- Researchers from Sweden used plasma C-peptide, HOMA-beta, HOMA-insulin resistance, glutamic-acid-decarboxylase autoantibodies (GADA), age of diagnosis, and BMI to classify patients into five clusters with different genetic profiles, which predicted early insulin requirement and incident CKD [105]. This clustering approach to classify diabetes has been verified in other populations including Asians [106]. In an ongoing 3-year randomized controlled trial (Precision medicine to Redefine Insulin Secretion and Monogenic diabetes [PRISM]), our group extended this concept and recruited 884 patients with young-onset diabetes aged less than 50 years who underwent structured clinical assessments and comprehensive biogenetic profiling, including measurement of HOMA-indices, C-peptide, and GADA to diagnose LADA and assess beta-cell function. These patients also had genome-wide genotyping to compute polygenic risk scores for beta-cell function and complications, targeted gene-sequencing to detect mutations of 34 genes for monogenic diabetes including MODY, and assessments of patient-reported outcome measures including psychosocial-behavioral factors and quality of life. Half of the patients were randomized to receive 1-year specialist-led multidisciplinary care in a diabetes centre, guided by their biogenetic profiles with counselling and support, aimed at attaining multiple treatment targets. After this 1-year multicomponent management [107,108], these patients will return to their usual clinics for follow-up with a yearly review at the diabetes centre, while the other half will receive usual care. All patients will undergo re-evaluation at 3 years to determine the primary outcome, defined by the incidence of all diabetes-related endpoints (https://clinicaltrials.gov/ct2/show/NCT04049149).
- In this implementation study, the analysis will be conducted according to the Reach, Effectiveness, Adoption, Implementation, and Evaluation (REAIM) framework [109] to inform planners, practitioners, and policymakers about the resources, infrastructure, personnel, logistics, and technology needed to bring about precision medicine in young-onset diabetes in clinical practice and assess its cost-effectiveness. Importantly, the establishment of this PRISM cohort will allow us to verify, modify, and develop risk algorithms for prognostication and treatment individualization. Meanwhile, the first-degree relatives of the trial participants are invited to undergo screening for diabetes/prediabetes using OGTT to complete an integrated detection-prevention-treatment program [110]. The creation of these cohorts, databases, and biobanks will provide valuable opportunities for multiomic and big data analysis to discover Asian-relevant novel common and rare variants supported by familial co-segregation analysis to confirm their validity and utility.
USING A MULTI-COMPONENT STRATEGY TO REDEFINE YOUNG-ONSET DIABETES FOR PRECISION CARE
- Establishing causal relationships is the most important principle in our pursuit of precision medicine through observations, experiments, and real-world practice [63,111,112]. In the case of young-onset diabetes, while many genetic, family, and life-course factors may not be avoided or changed, there are indeed many opportunities where nurture at a personal, family, and societal level can alter the disease trajectory and improve the outcome of these high-risk individuals [66,113,114]. According to the Pareto principle (or the “80 to 20 rule”), 80% of the effects in many events come from 20% of the causes [115]. Given our current understanding of the burden of young-onset diabetes, which affects one in five adult patients with diabetes in clinic settings in Asia, there is indeed strong justification to call for more investigations, investments, and interventions in these young patients and their family members which will bring long-term benefits not only to affected individuals and their families, but also to society at large.
CONCLUSIONS
-
CONFLICTS OF INTEREST
Juliana C.N. Chan holds patents for using genetic markers to predict diabetes and its complications for personalized care and is a co-founder of a start-up biotech company partially supported by the Technology Start-up Support Scheme for Universities (TSSSU) of the Hong Kong Government Innovation and Technology Commission to support precision care.
Article information
-
Acknowledgements
- We thank all staff, patients, and their relatives and the support of the Hong Kong Hospital Authority and the Hong Kong Genome Institute in the implementation of the Precision medicine to Redefine Insulin Secretion and Monogenic diabetes (PRISM) Project, supported by the Hong Kong Government Health and Medical Research Fund Commissioned Research (CFS-CUHK2).



- 1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30.PubMedPMC
- 2. Choi HH, Choi G, Yoon H, Ha KH, Kim DJ. Rising incidence of diabetes in young adults in South Korea: a national cohort study. Diabetes Metab J 2022;46:803–7.ArticlePubMedPMCPDF
- 3. Zhang X, Wu H, Fan B, Shi M, Lau ES, Yang A, et al. Lifetime risk of developing diabetes in Chinese people with normoglycemia or prediabetes: a modeling study. PLoS Med 2022;19:e1004045.ArticlePubMedPMC
- 4. Kim SM, Lee G, Choi S, Kim K, Jeong SM, Son JS, et al. Association of early-onset diabetes, prediabetes and early glycaemic recovery with the risk of all-cause and cardiovascular mortality. Diabetologia 2020;63:2305–14.ArticlePubMedPDF
- 5. Yang JJ, Yu D, Wen W, Saito E, Rahman S, Shu XO, et al. Association of diabetes with all-cause and cause-specific mortality in Asia: a pooled analysis of more than 1 million participants. JAMA Netw Open 2019;2:e192696.ArticlePubMedPMC
- 6. Chan JC, So W, Ma RC, Tong PC, Wong R, Yang X. The complexity of vascular and non-vascular complications of diabetes: the Hong Kong diabetes registry. Curr Cardiovasc Risk Rep 2011;5:230–9.ArticlePubMedPMC
- 7. Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, et al. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med 2014;127:616–24.ArticlePubMed
- 8. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41.ArticlePubMedPMC
- 9. Chan JC, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021;396:2019–82.PubMed
- 10. Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, et al. Current insights and emerging trends in earlyonset type 2 diabetes. Lancet Diabetes Endocrinol 2023;11:768–82.ArticlePubMed
- 11. Fan Y, Lau ES, Wu H, Yang A, Chow E, So WY, et al. Incidence of long-term diabetes complications and mortality in youth-onset type 2 diabetes: a systematic review. Diabetes Res Clin Pract 2022;191:110030.ArticlePubMed
- 12. Nanayakkara N, Curtis AJ, Heritier S, Gadowski AM, Pavkov ME, Kenealy T, et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia 2021;64:275–87.ArticlePubMedPMCPDF
- 13. Looker HC, Chang DC, Baier LJ, Hanson RL, Nelson RG. Diagnostic criteria and etiopathogenesis of type 2 diabetes and its complications: lessons from the Pima Indians. Presse Med 2023;52:104176.ArticlePubMed
- 14. Laakso M, Pyorala K. Age of onset and type of diabetes. Diabetes Care 1985;8:114–7.ArticlePubMedPDF
- 15. Chan JC, Cheung CK, Swaminathan R, Nicholls MG, Cockram CS. Obesity, albuminuria and hypertension among Hong Kong Chinese with non-insulin-dependent diabetes mellitus (NIDDM). Postgrad Med J 1993;69:204–10.ArticlePubMedPMCPDF
- 16. Ng MC, Lee SC, Ko GT, Li JK, So WY, Hashim Y, et al. Familial early-onset type 2 diabetes in Chinese patients: obesity and genetics have more significant roles than autoimmunity. Diabetes Care 2001;24:663–71.PubMed
- 17. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–40.ArticlePubMed
- 18. Rushforth NB, Bennett PH, Steinberg AG, Burch TA, Miller M. Diabetes in the Pima Indians: evidence of bimodality in glucose tolerance distributions. Diabetes 1971;20:756–65.ArticlePubMedPDF
- 19. Qiao Q, Nakagami T, Tuomilehto J, Borch-Johnsen K, Balkau B, Iwamoto Y, et al. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia 2000;43:1470–5.ArticlePubMedPDF
- 20. Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Effect of ethnicity on HbA1c levels in individuals without diabetes: systematic review and meta-analysis. PLoS One 2017;12:e0171315.ArticlePubMedPMC
- 21. Bergman M, Abdul-Ghani M, Neves JS, Monteiro MP, Medina JL, Dorcely B, et al. Pitfalls of HbA1c in the diagnosis of diabetes. J Clin Endocrinol Metab 2020;105:2803–11.ArticlePubMedPMCPDF
- 22. Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ, et al. Global prevalence of prediabetes. Diabetes Care 2023;46:1388–94.PubMed
- 23. Ko GT, Chan JC, Chan AW, Wong PT, Hui SS, Tong SD, et al. Low levels of awareness of suboptimal health conditions in a high-risk working population: the “better health for better Hong Kong” health promotion campaign. Int J Behav Med 2007;14:63–9.ArticlePubMedPDF
- 24. Li JK, Ng MC, So WY, Chiu CK, Ozaki R, Tong PC, et al. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab Res Rev 2006;22:46–52.ArticlePubMed
- 25. Zhang Y, Luk AO, Chow E, Ko GT, Chan MH, Ng M, et al. High risk of conversion to diabetes in first-degree relatives of individuals with young-onset type 2 diabetes: a 12-year follow-up analysis. Diabet Med 2017;34:1701–9.ArticlePubMedPDF
- 26. Sathish T, Khunti K, Narayan KM, Mohan V, Davies MJ, Yates T, et al. Effect of conventional lifestyle interventions on type 2 diabetes incidence by glucose-defined prediabetes phenotype: an individual participant data meta-analysis of randomized controlled trials. Diabetes Care 2023;46:1903–7.ArticlePubMedPDF
- 27. Bergman M, Buysschaert M, Ceriello A, Hussain A, Mohan V, Sesti G, et al. Current diagnostic criteria identify risk for type 2 diabetes too late. Lancet Diabetes Endocrinol 2023;11:224–6.ArticlePubMed
- 28. Herman WH, Ye W. Precision prevention of diabetes. Diabetes Care 2023;46:1894–6.ArticlePubMedPDF
- 29. Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, et al. Impaired glucose tolerance as a disorder of insulin action: longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988;318:1217–25.ArticlePubMed
- 30. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and metaanalysis. Diabetes Care 2013;36:1789–96.PubMedPMC
- 31. Yabe D, Seino Y. Type 2 diabetes via β-cell dysfunction in east Asian people. Lancet Diabetes Endocrinol 2016;4:2–3.ArticlePubMed
- 32. Chow EY, Chan JC. Insulin resistance versus β-cell dysfunction in type 2 diabetes: where public and personalised health meet. Lancet Diabetes Endocrinol 2020;8:92–3.ArticlePubMed
- 33. Ohn JH, Kwak SH, Cho YM, Lim S, Jang HC, Park KS, et al. 10-Year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol 2016;4:27–34.ArticlePubMed
- 34. Fan Y, Fan B, Lau ES, Lim CK, Wu H, Ma RC, et al. Comparison of beta-cell function between Hong Kong Chinese with young-onset type 2 diabetes and late-onset type 2 diabetes. Diabetes Res Clin Pract 2023;205:110954.ArticlePubMed
- 35. Chan JC, Lim LL, Luk AO, Ozaki R, Kong AP, Ma RC, et al. From Hong Kong diabetes register to JADE program to RAMP-DM for data-driven actions. Diabetes Care 2019;42:2022–31.ArticlePubMedPDF
- 36. Ke C, Stukel TA, Shah BR, Lau E, Ma RC, So WY, et al. Age at diagnosis, glycemic trajectories, and responses to oral glucose-lowering drugs in type 2 diabetes in Hong Kong: a population-based observational study. PLoS Med 2020;17:e1003316.ArticlePubMedPMC
- 37. Barnett AH, Eff C, Leslie RD, Pyke DA. Diabetes in identical twins: a study of 200 pairs. Diabetologia 1981;20:87–93.ArticlePubMedPDF
- 38. Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev 1990;6:1–27.ArticlePubMed
- 39. Sakul H, Pratley R, Cardon L, Ravussin E, Mott D, Bogardus C. Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. Am J Hum Genet 1997;60:651–6.PubMedPMC
- 40. Almgren P, Lehtovirta M, Isomaa B, Sarelin L, Taskinen MR, Lyssenko V, et al. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 2011;54:2811–9.ArticlePubMedPDF
- 41. Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature 2016;536:41–7.PubMedPMC
- 42. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281:64–91.ArticlePubMedPMCPDF
- 43. Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature 2020;582:240–5.PubMedPMC
- 44. Noordam R, Lall K, Smit RA, Laisk T; Estonian Biobank Research Team; Metspalu A, et al. Stratification of type 2 diabetes by age of diagnosis in the UK biobank reveals subgroup-specific genetic associations and causal risk profiles. Diabetes 2021;70:1816–25.ArticlePubMedPMCPDF
- 45. Christiansen CE, Arathimos R, Pain O, Molokhia M, Bell JT, Lewis CM. Stratified genome-wide association analysis of type 2 diabetes reveals subgroups with genetic and environmental heterogeneity. Hum Mol Genet 2023;32:2638–45.ArticlePubMedPMCPDF
- 46. Srinivasan S, Chen L, Todd J, Divers J, Gidding S, Chernausek S, et al. The first genome-wide association study for type 2 diabetes in youth: the Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes 2021;70:996–1005.ArticlePubMedPMCPDF
- 47. Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia 2017;60:769–77.ArticlePubMedPMCPDF
- 48. Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature 2019;570:71–6.PubMedPMC
- 49. Locke JM, Saint-Martin C, Laver TW, Patel KA, Wood AR, Sharp SA, et al. The common HNF1A variant I27L is a modifier of age at diabetes diagnosis in individuals with HNF1A-MODY. Diabetes 2018;67:1903–7.ArticlePubMedPMCPDF
- 50. Kettunen JL, Rantala E, Dwivedi OP, Isomaa B, Sarelin L, Kokko P, et al. A multigenerational study on phenotypic consequences of the most common causal variant of HNF1A-MODY. Diabetologia 2022;65:632–43.ArticlePubMedPMCPDF
- 51. Zhou K, Donnelly LA, Morris AD, Franks PW, Jennison C, Palmer CN, et al. Clinical and genetic determinants of progression of type 2 diabetes: a DIRECT study. Diabetes Care 2014;37:718–24.ArticlePubMedPMCPDF
- 52. Jiang G, Luk AO, Tam CH, Lau ES, Ozaki R, Chow EY, et al. Obesity, clinical, and genetic predictors for glycemic progression in Chinese patients with type 2 diabetes: a cohort study using the Hong Kong Diabetes Register and Hong Kong Diabetes Biobank. PLoS Med 2020;17:e1003209.ArticlePubMedPMC
- 53. Ma RC, Lee HM, Lam VK, Tam CH, Ho JS, Zhao HL, et al. Familial young-onset diabetes, pre-diabetes and cardiovascular disease are associated with genetic variants of DACH1 in Chinese. PLoS One 2014;9:e84770.ArticlePubMedPMC
- 54. Ma RC, Hu C, Tam CH, Zhang R, Kwan P, Leung TF, et al. Genome-wide association study in a Chinese population identifies a susceptibility locus for type 2 diabetes at 7q32 near PAX4. Diabetologia 2013;56:1291–305.PubMedPMC
- 55. Lam VK, Ma RC, Lee HM, Hu C, Park KS, Furuta H, et al. Genetic associations of type 2 diabetes with islet amyloid polypeptide processing and degrading pathways in Asian populations. PLoS One 2013;8:e62378.ArticlePubMedPMC
- 56. Pettitt DJ, Lawrence JM, Beyer J, Hillier TA, Liese AD, Mayer-Davis B, et al. Association between maternal diabetes in utero and age at offspring’s diagnosis of type 2 diabetes. Diabetes Care 2008;31:2126–30.ArticlePubMedPMCPDF
- 57. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–11.ArticlePubMedPDF
- 58. Tam WH, Ma RC, Ozaki R, Li AM, Chan MH, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017;40:679–86.ArticlePubMedPMCPDF
- 59. Li LJ, Huang L, Tobias DK, Zhang C. Gestational diabetes mellitus among Asians: a systematic review from a population health perspective. Front Endocrinol (Lausanne) 2022;13:840331.ArticlePubMedPMC
- 60. Mazidi M, Banach M, Kengne AP; Lipid and Blood Pressure Meta-analysis Collaboration Group. Prevalence of childhood and adolescent overweight and obesity in Asian countries: a systematic review and meta-analysis. Arch Med Sci 2018;14:1185–203.ArticlePubMedPMC
- 61. Ozaki R, Qiao Q, Wong GW, Chan MH, So WY, Tong PC, et al. Overweight, family history of diabetes and attending schools of lower academic grading are independent predictors for metabolic syndrome in Hong Kong Chinese adolescents. Arch Dis Child 2007;92:224–8.ArticlePubMedPMC
- 62. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59.ArticlePubMed
- 63. Chan JC, Chow E, Kong A, Cheung E, O T, Lim C, et al. Multifaceted nature of young-onset diabetes: can genomic medicine improve the precision of diagnosis and management? J Transl Genet Genom 2024;8:13–34.Article
- 64. Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol 2014;2:935–43.ArticlePubMed
- 65. Luk AO, Lau ES, Lim C, Kong AP, Chow E, Ma RC, et al. Diabetes-related complications and mortality in patients with young-onset latent autoimmune diabetes: a 14-year analysis of the prospective Hong Kong diabetes register. Diabetes Care 2019;42:1042–50.ArticlePubMedPDF
- 66. Ng MC, Li JK, So WY, Critchley JA, Cockram CS, Bell GI, et al. Nature or nurture: an insightful illustration from a Chinese family with hepatocyte nuclear factor-1 alpha diabetes (MODY3). Diabetologia 2000;43:816–8.ArticlePubMedPDF
- 67. Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care 2001;24:1522–7.PubMed
- 68. Yang A, Wu H, Lau ES, Ma RC, Kong AP, So WY, et al. Trends in glucose-lowering drug use, glycemic control, and severe hypoglycemia in adults with diabetes in Hong Kong, 2002-2016. Diabetes Care 2020;43:2967–74.ArticlePubMedPDF
- 69. Yang A, Wu H, Lau ES, Zhang X, Shi M, Fan B, et al. Glucose-lowering drug use, glycemic outcomes, and severe hypoglycemia: 18-year trends in 0.9 million adults with diabetes in Hong Kong (2002-2019). Lancet Reg Health West Pac 2022;26:100509.ArticlePubMedPMC
- 70. Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health 2005;95 Suppl 1:S144–50.ArticlePubMed
- 71. Dagogo-Jack S. Diabetes mellitus in developing countries and underserved communities. Cham: Switzerland Springer International Publishing; 2016. Chapter 5, Diabetes in China and the Western Pacific Region; p. 63-84 [cited 2024 Mar 22]. Available from: https://link.springer.com/chapter/10.1007/978-3-319-41559-8_5.
- 72. McCarthy M, Birney E. Personalized profiles for disease risk must capture all facets of health. Nature 2021;597:175–7.ArticlePubMedPDF
- 73. Chung WK, Erion K, Florez JC, Hattersley AT, Hivert MF, Lee CG, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:1617–35.ArticlePubMedPMCPDF
- 74. Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–6.ArticlePubMed
- 75. Hoy WE, Mathews JD, McCredie DA, Pugsley DJ, Hayhurst BG, Rees M, et al. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int 1998;54:1296–304.ArticlePubMed
- 76. Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int 2000;58:302–11.ArticlePubMed
- 77. Yokoyama H, Okudaira M, Otani T, Watanabe C, Takaike H, Miuira J, et al. High incidence of diabetic nephropathy in early-onset Japanese NIDDM patients: risk analysis. Diabetes Care 1998;21:1080–5.ArticlePubMedPDF
- 78. Luk AO, Lau ES, So WY, Ma RC, Kong AP, Ozaki R, et al. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care 2014;37:149–57.ArticlePubMedPDF
- 79. Lee ET, Keen H, Bennett PH, Fuller JH, Lu M. Follow-up of the WHO Multinational Study of Vascular Disease in Diabetes: general description and morbidity. Diabetologia 2001;44 Suppl 2:S3–13.ArticlePubMedPDF
- 80. Luk AO, Ke C, Lau ES, Wu H, Goggins W, Ma RC, et al. Secular trends in incidence of type 1 and type 2 diabetes in Hong Kong: a retrospective cohort study. PLoS Med 2020;17:e1003052.ArticlePubMedPMC
- 81. Leung CB, Cheung WL, Li PK. Renal registry in Hong Kong-the first 20 years. Kidney Int Suppl (2011) 2015;5:33–8.ArticlePubMedPMC
- 82. Lee J, Lee SH, Yoon KH, Cho JH, Han K, Yang Y. Risk of developing chronic kidney disease in young-onset type 2 diabetes in Korea. Sci Rep 2023;13:10100.ArticlePubMedPMCPDF
- 83. Wu H, Lau ES, Yang A, Fan B, Ma RC, Kong AP, et al. Young age at diabetes diagnosis amplifies the effect of diabetes duration on risk of chronic kidney disease: a prospective cohort study. Diabetologia 2021;64:1990–2000.ArticlePubMedPDF
- 84. de Boer IH, Caramori ML, Chan JC, Heerspink HJ, Hurst C, Khunti K, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int 2020;98:839–48.ArticlePubMed
- 85. Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation 2023;148:1606–35.ArticlePubMed
- 86. Wu H, Lau ES, Yang A, Zhang X, Ma RC, Kong AP, et al. Data resource profile: the Hong Kong Diabetes Surveillance Database (HKDSD). Int J Epidemiol 2022;51:e9–17.ArticlePubMedPDF
- 87. Ke C, Lau E, Shah BR, Stukel TA, Ma RC, So WY, et al. Excess burden of mental illness and hospitalization in young-onset type 2 diabetes: a population-based cohort study. Ann Intern Med 2019;170:145–54.ArticlePubMed
- 88. Wu H, Yang A, Lau ES, Zhang X, Fan B, Shi M, et al. Ageand sex-specific hospital bed-day rates in people with and without type 2 diabetes: a territory-wide population-based cohort study of 1.5 million people in Hong Kong. PLoS Med 2023;20:e1004261.ArticlePubMedPMC
- 89. Wu H, Lau ES, Ma RC, Kong AP, Wild SH, Goggins W, et al. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001-2016: a retrospective cohort study. Diabetologia 2020;63:757–66.ArticlePubMedPDF
- 90. Ke C, Shah BR, Luk AO, Di Ruggiero E, Chan JC. Cardiovascular outcomes trials in type 2 diabetes: time to include young adults. Diabetes Obes Metab 2020;22:3–5.ArticlePubMedPDF
- 91. Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC, et al. High prevalence of depressive symptoms in patients with type 1 and type 2 diabetes in developing countries: results from the International Diabetes Management Practices Study. Diabetes Care 2021;44:1100–7.ArticlePubMedPMCPDF
- 92. Seng JJ, Kwan YH, Lee VS, Tan CS, Zainudin SB, Thumboo J, et al. Differential health care use, diabetes-related complications, and mortality among five unique classes of patients with type 2 diabetes in Singapore: a latent class analysis of 71,125 patients. Diabetes Care 2020;43:1048–56.ArticlePubMedPMCPDF
- 93. Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–13.PubMedPMC
- 94. Ramos EL, Dayan CM, Chatenoud L, Sumnik Z, Simmons KM, Szypowska A, et al. Teplizumab and β-cell function in newly diagnosed type 1 diabetes. N Engl J Med 2023;389:2151–61.ArticlePubMed
- 95. Zhang L, Zhang Y, Shen S, Wang X, Dong L, Li Q, et al. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation: a multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2023;11:567–77.PubMed
- 96. Herman WH. The cost-effectiveness of diabetes prevention: results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clin Diabetes Endocrinol 2015;1:9.ArticlePubMedPMCPDF
- 97. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.ArticlePubMed
- 98. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–55.ArticlePubMed
- 99. Wu H, Yang A, Lau ES, Zhang X, Fan B, Ma RC, et al. 1-Year weight change after diabetes diagnosis and long-term incidence and sustainability of remission of type 2 diabetes in real-world settings in Hong Kong: an observational cohort study. PLoS Med 2024;21:e1004327.ArticlePubMedPMC
- 100. Chan JC, Paldanius PM, Mathieu C, Stumvoll M, Matthews DR, Del Prato S. Early combination therapy delayed treatment escalation in newly diagnosed young-onset type 2 diabetes: a subanalysis of the VERIFY study. Diabetes Obes Metab 2021;23:245–51.PubMed
- 101. Cheung JT, Yang A, Wu H, Lau ES, Kong AP, Ma RC, et al. Early treatment with dipeptidyl-peptidase 4 inhibitors reduces glycaemic variability and delays insulin initiation in type 2 diabetes: a propensity score-matched cohort study. Diabetes Metab Res Rev 2024;40:e3711.PubMed
- 102. Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:951–64.PubMed
- 103. Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–76.ArticlePubMed
- 104. Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care 2019;42:416–26.PubMed
- 105. Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361–9.ArticlePubMed
- 106. Ke C, Narayan KM, Chan JC, Jha P, Shah BR. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol 2022;18:413–32.ArticlePubMedPMCPDF
- 107. Lim LL, Lau ES, Kong AP, Davies MJ, Levitt NS, Eliasson B, et al. Aspects of multicomponent integrated care promote sustained improvement in surrogate clinical outcomes: a systematic review and meta-analysis. Diabetes Care 2018;41:1312–20.ArticlePubMedPMCPDF
- 108. Chan JC, Thewjitcharoen Y, Nguyen TK, Tan A, Chia YC, Hwu CM, et al. Effect of a web-based management guide on risk factors in patients with type 2 diabetes and diabetic kidney disease: a JADE randomized clinical trial. JAMA Netw Open 2022;5:e223862.ArticlePubMedPMC
- 109. Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health 2019;7:64.ArticlePubMedPMC
- 110. Kong AP, Luk AO, Chan JC. Detecting people at high risk of type 2 diabetes: how do we find them and who should be treated? Best Pract Res Clin Endocrinol Metab 2016;30:345–55.ArticlePubMed
- 111. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300.ArticlePubMedPMCPDF
- 112. Basu R. Precision medicine in diabetes: a multidisciplinary approach to an emerging paradigm. Cham: Springer; 2022. Chapter 5, Implementation of precision genetic approaches for type 1 and 2 diabetes; p. 111-29 [cited 2024 Mar 22]. Available from: https://link.springer.com/chapter/10.1007/978-3-030-98927-9_5.
- 113. Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care 2009;32:977–82.PubMedPMC
- 114. Chan JC, Sui Y, Oldenburg B, Zhang Y, Chung HH, Goggins W, et al. Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med 2014;174:972–81.ArticlePubMed
- 115. Naoum V, Kyriopoulos D, Charonis A, Athanasakis K, Kyriopoulos J. The Pareto principle (“80− 20 Rule”) in healthcare services in Greece. Value Health 2016;19:A618.Article
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite